Many people following a fat loss program have experienced the frustrating phenomenon that as they continue to lose bodyweight and approach a lower body fat percentage, fat loss from the midsection begins to halt and the body begins prioritizing weight loss from areas of the body other than the abdomen.
Why is belly fat so “stubborn”?
There are a few anatomical intricacies that make certain areas of fat in the body more stubborn and resilient to fat loss (2). The belly region is the area that is most frequently experienced to be “stubborn”, but some people can have stubborn fat in other areas as well.
The first and primary problem is that “stubborn fat” is higher in alpha-2 adrenoreceptors (3-7), and alpha-2 adrenoreceptors are more resilient to fat loss (8-13) due to the increased amount of adrenaline that must be present in order for them to stimulate fat mobilization.
This is summarized nicely by Lyle McDonald (1, page 51):
“Beta-2-receptors can be thought of as ‘good’ receptors, increasing lipolysis and adipose tissue blood flow. In contrast, alpha-2-receptors are distinctly bad, inhibiting lipolyisis and adipose tissue blood flow… Different areas of body fat have different distributions of alpha-2 and beta-2 adrenoreceptors and this profoundly affects how well or poorly fat can be mobilized or transported out of the fat cell…. This is clearly part of why stubborn fat is so stubborn, the normal lipolytic stimuli that should mobilize fatty acids don’t work effectively”
The second problem with stubborn fat is the lack of blood flow. Adipose tissue must have adequate blood flow for optimal fat loss (14-17), and stubborn fat receives much less blood flow than other areas of fat storage (18-19).
According to Lyle McDonald (1, page 52):
“Certain fat depots have significantly poorer blood flow than others… it means that blood borne hormones… can’t get to the fat cells. Second, poor blood flow makes it harder to get mobilized fat away from the fat cell so that it can be burned elsewhere.”
For all of these reasons, many people find that when they enter a caloric deficit, they lose weight from other areas of the body but the abdomen might remain relatively unchanged.
Thankfully, however, multiple steps can be taken to circumvent these obstacles and preferentially target fat loss from stubborn areas.
Strategy #1: Targeted Core/Cardio Supersets
For many years, it has been commonly accepted knowledge in the fitness industry that the concept of “spot reduction” — the idea that you can preferentially target certain areas of the body to lose fat from — is not possible.
Multiple studies have been conducted that have supported this belief.
A study by Kostek et al (20) involved 104 subjects who performed strength training on only one arm. After 12 weeks, an MRI was conducted to determine if the subjects had lost more fat from the trained arm compared to the untrained arm. Unfortunately, the amount of fat loss in the trained arm was no greater than the amount of fat loss in the untrained arm.
A similar study was conducted by Ramirez-Campillo R et al (21). Subjects trained only one leg during the 12 week program. Not only did the subjects fail to lose more fat in the trained leg than the untrained leg, they actually lost more fat in the upper body than in the lower body.
Therefore, these studies suggest that spot reduction must be impossible. Case closed, right?
Actually, not necessarily. To understand why spot reduction could still be possible, we must first understand the physiology of fat loss.
Physiology of Fat Loss
In order to lose fat, two steps must occur:
- 1 – Fat Mobilization
Fat is stored in adipose tissue cells, and the first step that must occur is to get the fat out of the fat cells and into the bloodstream.
It turns out that when it comes to fat mobilization, high-intensity exercise, such as weightlifting or sprinting, produces a larger output of adrenaline, which increases capacity for fat mobilization (22-26).
Unfortunately, however, as exercise intensity increases and adrenaline output is elevated, this simultaneously leads to a decrease in the second step of fat loss:
- 2 – Fat Burning
It’s not enough for the fat to simply make it out of the adipose tissue into the bloodstream. After the fat is mobilized, this energy must then be burned for fuel. It turns out that while high-intensity exercise is best for fat mobilization, low-intensity exercise is vastly superior for fat burning (27-29).
During high-intensity activities, the body often ends up burning glucose for fuel rather than fats. Therefore, it’s possible that in the previously cited studies on spot reduction, although the weight-lifting may have been mobilizing some of the fat from the trained limbs, those fatty acids were never being burned off and were simply returning to fat storage.
Therefore, we could hypothesize that in order to effectively spot reduce, first we would need to train the muscle using high-intensity activities such as weight-lifting, and then we would need to immediately follow up with some low-intensity cardio in order to burn the fat that was recently mobilized.
In fact, this is exactly what a fascinating study by di Palumbo et al (30) found.
Half of the subjects performed upper body weight-lifting immediately followed by 30 minutes of cardio. The other half of the subjects performed lower body weight-lifting immediately followed by 30 minutes of cardio.
Both groups lost the same amount of total fat over the course of 8 weeks, but skinfold measurements showed that the upper body training group lost more fat from their upper body while the lower body training group lost more fat from their lower body.
This is not particularly surprising; performing strength training immediately prior to cardio has been shown to increase fat mobilization during cardio (31), and this effect appears to be greatest in areas adjacent to contracting muscles (32).
Therefore, these findings are in line with fat loss physiology and provide evidence that in the proper context, spot reduction could very well be possible.
Spot Reduction Supersets
In order for spot reduction to occur, all three of the following objectives need to be achieved:
- Increase adrenaline output
- Direct blood flow to the target area
- Burn the mobilized fatty acids as fuel
In order to do this, I recommend starting with an abs/sprint superset in order to spike adrenaline with the sprints and then immediately contract the muscles of the abs so that the adrenaline-filled blood rushes to the site of muscular contractions.
After a few rounds of this, immediately follow with low-intensity cardio so that the fatty acids that were mobilized into the bloodstream can now be burned as fuel.
For example:

Which Exercises Are Best?
There are numerous ab exercises that can be part of a well-rounded core training plan (see here for more info on ab training), but for spot reduction purposes, I would focus on exercises that isolate the core such as ab rollouts, hollow body holds, and deadbugs. If you want to target the sides of the core, you could also include side planks.
For cardio, I prefer doing the sprints on the treadmill, but the bike is also a good choice. Alternatively, if treadmill and bike are not an option, jump rope works well. Other forms of cardio, such as burpees, battle ropes, etc. could also work, but in general I think that the sprints or jump ropes would be optimal.
For the low-intensity cardio, I would recommend light jogging or walking on an incline. Alternatively, stairmaster or elliptical could be used. As long as the heart rate stays in the 130-140 BPM range, any of these pieces of equipment can do the job.
For the purposes of this program, I would recommend completing the core training in the morning with the cardio, and then do your normal upper body and lower body strength training in the afternoon/evening. If this is not possible, then I would recommend doing your abs/sprints first in your workout, then your low-intensity cardio second, and then end your workout with your upper and lower body strength training after the abs/cardio work has been completed. This will help maximize the proportion of fat burning that is derived from the core area.
This spot reduction program does not have to be applied exclusively to the core. Instead of ab exercises, you could do bridges and band walks if you wanted to spot reduce from the glutes, or tricep pushdowns and bicep curls if you wanted to spot reduce from the arms. In my experience, however, belly fat tends to be the most difficult to lose, and therefore this routine is particularly useful for the core.
This is the foundation of a spot reduction program, but thankfully, additionally strategies are available to further increase our success:
Strategy #2: Increasing Adrenaline Output with Caffeine
The first step for losing fat from stubborn areas is to increase adrenaline output, as these regions require a higher quantity of adrenaline present in order for fat mobilization to occur.
One strategy that can be used to increase adrenaline output during exercise is caffeine ingestion (33-34). Caffeine increases the magnitude of the body’s fight-or-flight response, leading to higher adrenaline output. Studies have found that when subjects consume caffeine prior to exercise, fat mobilization is higher (35-36) and this consequently leads to increased fat-burning (35-36).
Green tea not only contains caffeine but is also high in beneficial compounds for health and therefore would be a great choice to consume prior to exercise. Alternatively, coffee or caffeine tablets could also suffice.
Strategy #3: Redirecting Blood Flow with Ab Belts
Once adrenaline has been produced from the adrenal glands, it is essential for the adrenaline to make it from the adrenal glands through the bloodstream to the site of fat storage.
As mentioned previously in this article, stubborn fat tends to have less blood flow than other regions (18-19), making this process more difficult.
It turns out that one way we can increase blood flow is by increasing temperature (37). We need a technique, however, that increases temperature in only the abdominal region without increasing temperature in the rest of the body.
One of the simplest ways to accomplish this is one of those gimmicky-looking ab wraps that you place around your waist.
This may look silly, but simply by increasing the temperature of the core, this should theoretically result in an increase in blood flow to the area which can facilitate fat mobilization.
In fact, a study by Moreira et al (38) had 19 subjects complete 2 cardio sessions per week over the course of 10 weeks. Half of the subjects performed the cardio with an ab wrap around their waist, while the other half completed the same amount of exercise without the wrap. At the end of the study, both groups decreased their body fat percentage by approximately 1.5%. The group that used the ab wrap, however, decreased their waist circumference by a greater magnitude and also showed an overall increased amount of fat loss in the abdominal region when measured by skinfold calipers and ultrasound equipment.
Not only does this study support the use of ab wraps, but it once again provides evidence that spot reduction is not impossible.
Strategy #4: Increase Adrenal Receptor Sensitivity with Yohimbine HCL
Once adrenaline has been produced and travels from the adrenal glands to the site of fat storage, it must stimulate the adrenal receptors to release fat into the bloodstream. Unfortunately, this is more difficult in regions of stubborn fat, as these areas contain more alpha-2 adrenoreceptors which require more adrenaline to be present.
Therefore, anything that could block the alpha-2-adrenoceptors would mean that less adrenaline would need to be present and would cause stubborn fat to behave in a more similar manner to other fat areas from the rest of the body.
It turns out that a supplement called Yohimbine HCL does exactly that. By blocking alpha-2 receptors, Yohimbine HCL allows for less adrenaline to be needed in order for fat mobilization to occur. Taking yohimbine prior to exercise has been demonstrated to increase the amount of fat that is mobilized from the adipose tissue into the bloodstream (40-44).

Yohimbine Must Be Taken While Fasted
In order for yohimbine to work, however, it must be taken in a fasted state (45).
Cycling Yohimbine
According to a study by Galitzky et al (46), the body begins to build up a tolerance to yohimbine after two weeks of consumption. Therefore, this supplement is best used for only 1-2 weeks at a time during a Cutting phase, and then halted for a few weeks during a Maintenance phase (more information on the interplay between Maintenance and Cutting phases can be found here).
Side Effects of Yohimbine
Yohimbine is a stimulant, and like all stimulants, there is the potential for side effects, such as increased blood pressure, stomach irritation, or anxiety.
These effects should diminish once consumption is ceased. That being said, if you are currently taking any medication, you should check with your doctor to make sure that yohimbine will not have any adverse interactions with your current medicine.
Yohimbine Dosage
Dosages of 0.2mg/kg bodyweight have been successfully used to increase fat burning without significant implications on cardiovascular parameters like heart rate and blood pressure. This results in a dosage of:
14 mg for a 150lb person
18 mg for a 200lb person
22 mg for a 250lb person
If you have never used yohimbine before, I recommend testing it out in incremental dosages. For example, if your target dosage is 15 mg, first try 5 mg to make sure there are no side effects. If 5 mg is tolerable, give 10 mg a try. If 10 mg is okay, then try increasing to 15 mg.
Strategy #5: Increase Fat-Burning with Fasted Cardio
Once the fat has been mobilized out of storage into the bloodstream, it must then be burned as fuel. One strategy that has often been theorized to increase fat-burning is performing cardio in a fasted state.
In general, spending a prolonged period of time each day in a fasted state appears to be beneficial for health. Fasting facilitates the adenosine monophosphate-activated protein kinase (AMPK) pathway and stimulates autophagy (47), which is one of our body’s prominent mechanisms for repair.
When it comes to exercise, however, fasted cardio tends to get a bad rap. Multiple studies have found that performing cardio in a fasted state does not increase fat loss compared to performing cardio in a fed state (48-50).
These beliefs are succinctly summarized by Brad Shoenfeld:
“In conclusion, the literature does not support the efficacy of training early in the morning on an empty stomach as a tactic to reduce body fat. At best, the net effect on fat loss associated with such an approach will be no better than training after meal consumption, and quite possibly, it would produce inferior results.”
I am not disagreeing with the conclusions of these studies. Since fasted cardio does not burn more calories than fed cardio, there is no reason why it would lead to increased weight loss.
What I am interested in, however, is not whether fasted cardio leads to increased fat loss, but whether it can be a useful tool when using spot reduction techniques for preferentially targeting stubborn fat areas.
Overall, I believe it can.
Fasted Cardio for Spot Reduction and Stubborn Fat
If exercise is performed after eating, the bloodstream will be full of nutrients, and the body will burn these nutrients for fuel rather than mobilizing fat from the adipose tissue.
Normally, this would not be a problem for most people seeking fat loss. If you use the nutrients that are already in the bloodstream during exercise, there will be less available later, and the body will eventually begin mobilizing energy from the fat stores to make up for this caloric deficit.
In the case where we are trying to spot reduce specific fat regions during exercise, however, this becomes an issue. We need the fat to become mobilized during the abs/sprints superset so that it is available to be directly burned during the low-intensity cardio.
This is problematic if we are doing cardio in a fed state. When insulin rises after consuming a meal, this directly inhibits fat mobilization (51-58), preventing the fat from making it into the bloodstream.
When cardio is performed in a fasted state, however, blood flow to the abdominal region is elevated (59), fat mobilization increases (60-65), and fatty acid oxidation increases (66-75).
Therefore, I believe that although fasted cardio will not increase overall fat loss, it can be a useful tool when your goal is to use spot reduction techniques, particularly in stubborn fat regions.
Putting it All Together
Overall, the final spot reduction program would look as follows:

A sample program might look as follows:
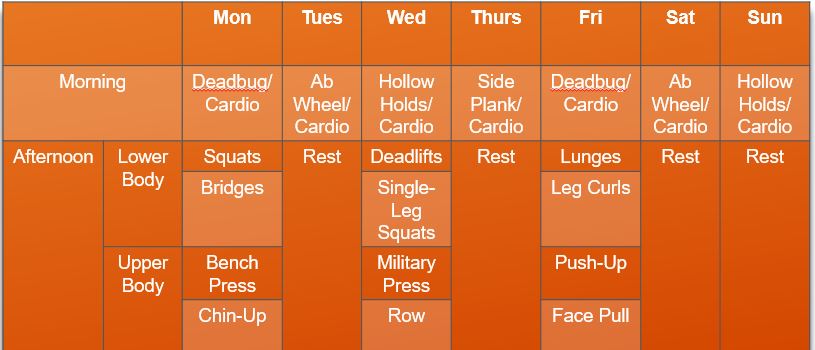
Are all of these steps necessary?
The foundation of this program is the ab/sprint superset followed by the low-intensity cardio. Doing the cardio while fasted and taking yohimbine should be considered added bonuses.
One possibility is that the fasted cardio/yohimbine will augment the training program, and superior results will be achieved compared to if the abs/cardio supersets were used alone.
Alternatively, another possibility is that these steps are redundant with each other and that if you are already implementing the abs/sprint superset, this should be sufficient for fat mobilization, and the fasted cardio/yohimbine is not necessary at this point.
Unfortunately, I have not seen any research that tested all of these variables simultaneously, so the only way to know is to experiment on yourself.
Who is this program meant for?
For many individuals, this program might be overkill. Many fitness enthusiasts are able to achieve satisfactory results without this level of physiological manipulation. This program is primarily intended for those individuals who have plateaued with their current progress and may benefit from more complex strategies for improving body composition outside of their normal exercise and dietary plans.
Conclusions
Losing fat from the abdominal region can be difficult and becomes increasingly more challenging for those who are already relatively lean.
Thankfully, contrary to popular belief, spot reduction is likely possible. By implementing advanced training techniques, more abdominal fat can be lost while spending less overall time in a calorie deficit.
Works Cited
1 McDonald, L. (2008). The stubborn fat solution. Salt Lake City: Lee McDonald, 93.
References
2 Monzon, J. R., Basile, R., Heneghan, S., Udupi, V., & Green, A. (2002). Lipolysis in adipocytes isolated from deep and superficial subcutaneous adipose tissue. Obesity research, 10(4), 266-269.
3 Mauriege, P., Galitzky, J., Berlan, M., & Lafontan, M. (1987). Heterogeneous distribution of beta and alpha‐2 adrenoceptor binding sites in human fat cells from various fat deposits: functional consequences. European journal of clinical investigation, 17(2), 156-165.
4 Hoffstedt, J., Arner, P., Hellers, G., & Lönnqvist, F. (1997). Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. Journal of lipid research, 38(4), 795-804
5 Arner, P. (1995). Differences in lipolysis between human subcutaneous and omental adipose tissues. Annals of medicine, 27(4), 435-438.
6 Arner, P., Kriegholm, E., Engfeldt, P., & Bolinder, J. (1990). Adrenergic regulation of lipolysis in situ at rest and during exercise. The Journal of clinical investigation, 85(3), 893-898.
7 Fain, J. N., & Garcĩa-Sáinz, J. A. (1983). Adrenergic regulation of adipocyte metabolism. Journal of lipid research, 24(8), 945-966.
8 Galitzky, J., Lafontan, M., Nordenström, J., & Arner, P. (1993). Role of vascular alpha-2 adrenoceptors in regulating lipid mobilization from human adipose tissue. The Journal of clinical investigation, 91(5), 1997-2003.
9 Hoffstedt, J., Arner, P., Hellers, G., & Lönnqvist, F. (1997). Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. Journal of lipid research, 38(4), 795-804.
10 Arner, P. (1995). Differences in lipolysis between human subcutaneous and omental adipose tissues. Annals of medicine, 27(4), 435-438.
11 Arner, P., Kriegholm, E., Engfeldt, P., & Bolinder, J. (1990). Adrenergic regulation of lipolysis in situ at rest and during exercise. The Journal of clinical investigation, 85(3), 893-898.
12 Wijnen, J. A. G., Van Baak, M. A., De Haan, C., Boudier, H. S., Tan, F. S., & Van Bortel, L. M. A. B. (1993). Beta-blockade and lipolysis during endurance exercise. European journal of clinical pharmacology, 45(2), 101-105.
13 Lönnqvist, F., Nyberg, B., Wahrenberg, H., & Arner, P. (1990). Catecholamine-induced lipolysis in adipose tissue of the elderly. The Journal of clinical investigation, 85(5), 1614-1621.
14 Bülow, J., & Madsen, J. (1981). Influence of blood flow on fatty acid mobilization from lipolytically active adipose tissue. Pflügers Archiv, 390(2), 169-174.
15 Galitzky, J., Lafontan, M., Nordenström, J., & Arner, P. (1993). Role of vascular alpha-2 adrenoceptors in regulating lipid mobilization from human adipose tissue. The Journal of clinical investigation, 91(5), 1997-2003.
16 Hjemdahl, P., & Fredholm, B. B. (1976). Influence of adipose tissue blood flow on the lipolytic response to circulating noradrenaline at normal and reduced pH. Acta Physiologica Scandinavica, 98(1), 74-79.
17 Frayn, K. N. (1999). Macronutrient metabolism of adipose tissue at rest and during exercise: a methodological viewpoint. Proceedings of the Nutrition Society, 58(4), 877-886.
18 Ardilouze, J. L., Karpe, F., Currie, J. M., Frayn, K. N., & Fielding, B. A. (2004). Subcutaneous adipose tissue blood flow varies between superior and inferior levels of the anterior abdominal wall. International journal of obesity, 28(2), 228.
19 Frayn, K. N. (1999). Macronutrient metabolism of adipose tissue at rest and during exercise: a methodological viewpoint. Proceedings of the Nutrition Society, 58(4), 877-886.
20 Kostek, M. A., Pescatello, L. S., Seip, R. L., Angelopoulos, T. J., Clarkson, P. M., Gordon, P. M., … & Hoffman, E. P. (2007). Subcutaneous fat alterations resulting from an upper-body resistance training program. Medicine & Science in Sports & Exercise, 39(7), 1177-1185.
21 Ramírez-Campillo, R., Andrade, D. C., Campos-Jara, C., Henríquez-Olguín, C., Alvarez-Lepín, C., & Izquierdo, M. (2013). Regional fat changes induced by localized muscle endurance resistance training. The Journal of Strength & Conditioning Research, 27(8), 2219-2224.
22 Purdom, T., Kravitz, L., Dokladny, K., & Mermier, C. (2018). Understanding the factors that effect maximal fat oxidation. Journal of the International Society of Sports Nutrition, 15(1), 3.
23 Horowitz, J. F., & Klein, S. (2000). Lipid metabolism during endurance exercise. The American journal of clinical nutrition, 72(2), 558S-563S.
24 Qvisth, V., Hagström-Toft, E., Enoksson, S., Moberg, E., Arner, P., & Bolinder, J. (2006). Human skeletal muscle lipolysis is more responsive to epinephrine than to norepinephrine stimulation in vivo. The Journal of Clinical Endocrinology & Metabolism, 91(2), 665-670.
25 Mora-Rodriguez, R., & Coyle, E. F. (2000). Effects of plasma epinephrine on fat metabolism during exercise: interactions with exercise intensity. American Journal of Physiology-Endocrinology And Metabolism, 278(4), E669-E676.
26 Samra, J. S., Simpson, E. J., Clark, M. L., Forster, C. D., Humphreys, S. M., Macdonald, I. A., & Frayn, K. N. (1996). Effects of epinephrine infusion on adipose tissue: interactions between blood flow and lipid metabolism. American Journal of Physiology-Endocrinology And Metabolism, 271(5), E834-E839.
27 Frayn, K. N. (1998). Regulation of fatty acid delivery in vivo. Advances in experimental medicine and biology, 441, 171.
28 Sidossis, L. S., Gastaldelli, A. M. A. L. I. A., Klein, S. A. M. U. E. L., & Wolfe, R. R. (1997). Regulation of plasma fatty acid oxidation during low-and high-intensity exercise. American Journal of Physiology-Endocrinology And Metabolism, 272(6), E1065-E1070.
29 Coyle, E. F. (1995). Substrate utilization during exercise in active people. The American journal of clinical nutrition, 61(4), 968S-979S.
30 di Palumbo Scotto, A., Guerra, E., Orlandi, C., Bazzucchi, I., & Sacchetti, M. (2017). Effect of combined resistance and endurance exercise training on regional fat loss. The Journal of sports medicine and physical fitness, 57(6), 794-801.
31 Goto, K., Ishii, N., Sugihara, S., Yoshioka, T., & Takamatsu, K. (2007). Effects of resistance exercise on lipolysis during subsequent submaximal exercise. Medicine and science in sports and exercise, 39(2), 308-315.
32 Stallknecht, B., Dela, F., & Helge, J. W. (2007). Are blood flow and lipolysis in subcutaneous adipose tissue influenced by contractions in adjacent muscles in humans?. American Journal of Physiology-Endocrinology and Metabolism, 292(2), E394-E399.
33 Van Soeren, M. H., Sathasivam, P., Spriet, L. L., & Graham, T. E. (1993). Caffeine metabolism and epinephrine responses during exercise in users and nonusers. Journal of Applied Physiology, 75(2), 805-812.
34 Norager, C. B., Jensen, M. B., Weimann, A., & Madsen, M. R. (2006). Metabolic effects of caffeine ingestion and physical work in 75‐year old citizens. A randomized, double‐blind, placebo‐controlled, cross‐over study. Clinical endocrinology, 65(2), 223-228.
35 Ryu, S., Choi, S. K., JoUNG, S. S., Suh, H., Cha, Y. S., Lee, S., & Lim, K. (2001). Caffeine as a lipolytic food component increases endurance performance in rats and athletes. Journal of nutritional science and vitaminology, 47(2), 139-146.
36 Kim, T. W., Shin, Y. O., Lee, J. B., Min, Y. K., & Yang, H. M. (2010). Effect of caffeine on the metabolic responses of lipolysis and activated sweat gland density in human during physical activity. Food Science and Biotechnology, 19(4), 1077-1081.
37 Astrup, A., Bülow, J., & Madsen, J. (1980). Skin temperature and subcutaneous adipose blood flow in man. Scandinavian journal of clinical and laboratory investigation, 40(2), 135-138.
38 Moreira, J. S., de Melo, A. S. C. P., Noites, A., Couto, M. F., de Melo, C. A., & de Almeida Adubeiro, N. C. F. (2013). Plaster body wrap: effects on abdominal fat. Integrative medicine research, 2(4), 151-156.
39 Hodgetts, V. A. N. E. S. S. A., Coppack, S. W., Frayn, K. N., & Hockaday, T. D. (1991). Factors controlling fat mobilization from human subcutaneous adipose tissue during exercise. Journal of Applied Physiology, 71(2), 445-451.
40 Galitzky, J., Taouis, M., Berlan, M., Riviere, D., Garrigues, M., & Lafontan, M. (1988). α2‐Antagonist compounds and lipid mobilization: evidence for a lipid mobilizing effect of oral yohimbine in healthy male volunteers. European journal of clinical investigation, 18(6), 587-594.
41 Lafontan, M., Berlan, M., Galitzky, J., & Montastruc, J. L. (1992). Alpha-2 adrenoceptors in lipolysis: α 2 antagonists and lipid-mobilizing strategies.
42 McCarty, M. F. (2002). Pre-exercise administration of yohimbine may enhance the efficacy of exercise training as a fat loss strategy by boosting lipolysis. Medical hypotheses, 58(6), 491-495.
43 Zahorska-Markiewicz, B., Kucio, C., & Piskorska, D. (1986). Adrenergic control of lipolysis and metabolic responses in obesity. Hormone and metabolic research, 18(10), 693-697.
44 Berlan, M., Galitzky, J., Riviere, D., Foureau, M., Tran, M. A., Flores, R., … & Lafontan, M. (1991). Plasma catecholamine levels and lipid mobilization induced by yohimbine in obese and non-obese women. International journal of obesity, 15(5), 305-315.
45 Galitzky, J., Taouis, M., Berlan, M., Riviere, D., Garrigues, M., & Lafontan, M. (1988). α2‐Antagonist compounds and lipid mobilization: evidence for a lipid mobilizing effect of oral yohimbine in healthy male volunteers. European journal of clinical investigation, 18(6), 587-594.
46 Galitzky, J., Riviere, D., Tran, M. A., Montastruc, J. L., & Berlan, M. (1990). Pharmacodynamic effects of chronic yohimbine treatment in healthy volunteers. European journal of clinical pharmacology, 39(5), 447-451.
47 Descamps, O., Riondel, J., Ducros, V., & Roussel, A. M. (2005). Mitochondrial production of reactive oxygen species and incidence of age-associated lymphoma in OF1 mice: effect of alternate-day fasting. Mechanisms of ageing and development, 126(11), 1185-1191.
48 Hackett, D., & Hagstrom, A. (2017). Effect of overnight fasted exercise on weight loss and body composition: a systematic review and meta-analysis. Journal of Functional Morphology and Kinesiology, 2(4), 43.
49 Schoenfeld, B. J., Aragon, A. A., Wilborn, C. D., Krieger, J. W., & Sonmez, G. T. (2014). Body composition changes associated with fasted versus non-fasted aerobic exercise. Journal of the International Society of Sports Nutrition, 11(1), 54.
50 Gillen, J. B., Percival, M. E., Ludzki, A., Tarnopolsky, M. A., & Gibala, M. J. (2013). Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity, 21(11), 2249-2255.
51 Moro, C., Pillard, F., De Glisezinski, I., Crampes, F., Thalamas, C., Harant, I., … & Berlan, M. (2007). Sex differences in lipolysis‐regulating mechanisms in overweight subjects: effect of exercise intensity. Obesity, 15(9), 2245-2255.
52 Frayn, K. N. (1998). Regulation of fatty acid delivery in vivo. In Skeletal Muscle Metabolism in Exercise and Diabetes (pp. 171-179). Springer, Boston, MA.
53 Jensen, M. D., Caruso, M., Heiling, V., & Miles, J. M. (1989). Insulin regulation of lipolysis in nondiabetic and IDDM subjects. Diabetes, 38(12), 1595-1601.
54 Bonadonna, R. C., Groop, L. C., Zych, K. A. T. H. L. E. E. N., Shank, M. Y. R. O. N., & DeFronzo, R. A. (1990). Dose-dependent effect of insulin on plasma free fatty acid turnover and oxidation in humans. American Journal of Physiology-Endocrinology And Metabolism, 259(5), E736-E750.
55 Frayn, K. N., Shadid, S. A. M. Y. A. H., Hamlani, R. O. O. H. I., Humphreys, S. M., Clark, M. L., Fielding, B. A., … & Coppack, S. W. (1994). Regulation of fatty acid movement in human adipose tissue in the postabsorptive-to-postprandial transition. American Journal of Physiology-Endocrinology And Metabolism, 266(3), E308-E317.
56 Cimmino, M., Agosto, A., Minaire, Y., & Geloen, A. (1995). In situ regulation of lipolysis by insulin and norepinephrine: a microdialysis study during euglycemic-hyperinsulinemic clamp. Metabolism, 44(12), 1513-1518.
57 Horowitz, J. F., Mora-Rodriguez, R., Byerley, L. O., & Coyle, E. F. (1997). Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. American Journal of Physiology-Endocrinology And Metabolism, 273(4), E768-E775.
58 Dimitriadis, G., Mitrou, P., Lambadiari, V., Maratou, E., & Raptis, S. A. (2011). Insulin effects in muscle and adipose tissue. Diabetes research and clinical practice, 93, S52-S59.
59 Engfeldt, P., & Linde, B. (1992). Subcutaneous adipose tissue blood flow in the abdominal and femoral regions in obese women: effect of fasting. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity, 16(11), 875-879.
60 De Glisezinski, I., Harant, I., Crampes, F., Trudeau, F., Felez, A., Cottet-Emard, J. M., … & Riviere, D. (1998). Effect of carbohydrate ingestion on adipose tissue lipolysis during long-lasting exercise in trained men. Journal of Applied Physiology, 84(5), 1627-1632.
61 Gjedsted, J., Gormsen, L. C., Nielsen, S., Schmitz, O., Djurhuus, C. B., Keiding, S., … & Møller, N. (2007). Effects of a 3‐day fast on regional lipid and glucose metabolism in human skeletal muscle and adipose tissue. Acta physiologica, 191(3), 205-216.
62 Horowitz, J. F., Mora-Rodriguez, R., Byerley, L. O., & Coyle, E. F. (1997). Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. American Journal of Physiology-Endocrinology And Metabolism, 273(4), E768-E775.
63 Horowitz, J. F., Mora-Rodriguez, R., Byerley, L. O., & Coyle, E. F. (1999). Substrate metabolism when subjects are fed carbohydrate during exercise. American Journal of Physiology-Endocrinology and Metabolism, 276(5), E828-E835.
64 Coyle, E. F. (1995). Substrate utilization during exercise in active people. The American journal of clinical nutrition, 61(4), 968S-979S.
65 Coppack, S. W., Evans, R. D., Fisher, R. M., Frayn, K. N., Gibbons, G. F., Humphreys, S. M., … & Hockaday, T. D. R. (1992). Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism, 41(3), 264-272.
66 Derave, W., Mertens, A., Muls, E., Pardaens, K., & Hespel, P. (2007). Effects of Post‐absorptive and Postprandial Exercise on Glucoregulation in Metabolic Syndrome. Obesity, 15(3), 704-711.
67 Achten, J., & Jeukendrup, A. E. (2004). Optimizing fat oxidation through exercise and diet. Nutrition, 20(7-8), 716-727.
68 Achten, J., & Jeukendrup, A. E. (2003). The effect of pre-exercise carbohydrate feedings on the intensity that elicits maximal fat oxidation. Journal of Sports Science, 21(12), 1017-1025.
69 Zoladz, J., Konturek, S., Duda, K., Majerczak, J., Sliwowski, Z., Grandys, M., & Bielanski, W. (2005). EFFECT OF MODERATE INCREMENTAL EXERCISE, PERFORMED. Journal of Physiology and Pharmacology, 56(1), 63-85.
70 Frawley, K., Greenwald, G., Rogers, R. R., Petrella, J. K., & Marshall, M. R. (2018). Effects of prior fasting on fat oxidation during resistance exercise. International journal of exercise science, 11(2), 827.
71 Iwayama, K., Kawabuchi, R., Park, I., Kurihara, R., Kobayashi, M., Hibi, M., … & Tokuyama, K. (2014). Transient energy deficit induced by exercise increases 24-h fat oxidation in young trained men. American Journal of Physiology-Heart and Circulatory Physiology.
72 Bachman, J. L., Deitrick, R. W., & Hillman, A. R. (2016). Exercising in the fasted state reduced 24-hour energy intake in active male adults. Journal of nutrition and metabolism, 2016.
73 Shimada, K., Yamamoto, Y., Iwayama, K., Nakamura, K., Yamaguchi, S., Hibi, M., … & Tokuyama, K. (2013). Effects of post-absorptive and postprandial exercise on 24 h fat oxidation. Metabolism, 62(6), 793-800.
74 Iwayama, K., Kurihara, R., Nabekura, Y., Kawabuchi, R., Park, I., Kobayashi, M., … & Tokuyama, K. (2015). Exercise increases 24-h fat oxidation only when it is performed before breakfast. EBioMedicine, 2(12), 2003-2009.
75 Vieira, A. F., Costa, R. R., Macedo, R. C. O., Coconcelli, L., & Kruel, L. F. M. (2016). Effects of aerobic exercise performed in fasted v. fed state on fat and carbohydrate metabolism in adults: a systematic review and meta-analysis. British Journal of Nutrition, 116(7), 1153-1164.
